Abstract
Introduction. Recent research in lymphoma has resulted in better outcomes for clinical trial populations. Population studies have suggested that some real-world patients (pts) have not benefited. We hypothesized that one reason for this discrepancy is the difference between trial participants and real-world pts. We aimed to: 1) Compare demographics and baseline clinical characteristics of real-world and clinical trial pts receiving first-line therapy for diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL); and 2) Compare demographics and baseline clinical characteristics of real-world DLBCL, FL, and MCL pts with clinical trial eligibility criteria.
Methods. Using ClinicalTrials.gov, we identified all phase 2 and 3 clinical trials that opened between 2002-2017 and included pts with DLBCL, FL, MCL. Published trials that included front-line immunotherapy and chemotherapy were selected, and eligibility criteria recorded. We reviewed publications and recorded pt numbers and characteristics. Using the Weill Cornell Medicine (WCM) Lymphoma Database, an IRB-approved, prospective cohort which started in 2010, we identified all pts diagnosed with DLBCL, FL, and MCL and recorded baseline characteristics. Descriptive statistics were used to describe clinical trial eligibility and pt characteristics. Fisher's exact test was used to compare pt characteristics.
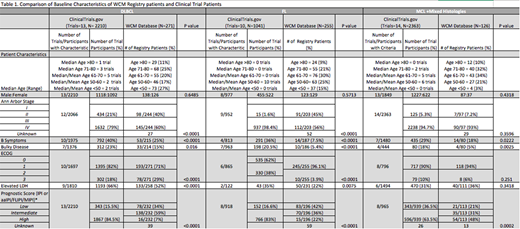
Results. We identified 642 phase 2 and 3 trials on Clinicaltrials.gov, 37 of which met predefined criteria. The most frequent exclusion criteria were HIV infection (n=33), pregnancy (n=25), HBV infection (n=21), history of non-lymphoma cancer (n=19), ECOG>2 (n=16), HCV infection (n=16), serum creatinine >2 mg/dL or >2x ULN (n=15), active infection (n=12), history of MI (n=11), serum bilirubin >2 mg/dL or >2x ULN (n=7), congestive heart failure (n=4), hemoglobin (Hb) <10g/dL (n=4). A total of 5614 pts were enrolled in 37 trials. Pt characteristics are listed in Table 1. Of 3690 enrolled in the 23 trials that reported the number of patients screened for eligibility, 502 (14%) were excluded based on eligibility criteria.
We identified 652 pts in the WCM Database with newly diagnosed DLBCL, FL, and MCL (Table 1). Key differences between clinical trial and Database populations for DLBCL included proportion of pts with stage 3-4 disease (79% vs 60%, p<0.001), presence of B symptoms (40% vs 25%, p<0.001) or bulky disease (23% vs 15%, p=0.016), and intermediate or high IPI (85% vs 66%, p<0.001); 36% of Database pts were age >70. Among FL pts, key differences between trial and Database populations included proportion with stage 3-4 disease (98% vs 56%, p<0.001), presence of B symptoms (36% vs 8%, p<0.001) or bulky disease (21% vs. 5%, p<0.001), and intermediate or high FLIPI (83% vs 58%, p<0.001). All FL trials had a median age between 50-60, whereas 30% Database pts varied in age from 27-93 years and 30% were age >70. Clinical trial vs. Database MCL pts differed in proportion with presence of B symptoms (29% vs 18%, p=0.022) or bulky disease (18% vs 5%, p=0.025), and intermediate or high MIPI (63.5% vs 79%, p=0.002); 42% of Database pts were age >70.
Of all 652 pts from the Database, 190 (29%) had characteristics that may have excluded them from clinical trial participation. The most common reasons for exclusion included history of cancer (11%), cardiac arrhythmias (7%), MI (6%), active infections (6%) and Hb <10g/dL (5%). Only 19 might have been excluded due to serum creatinine >2mg/dL (1.4%), serum bilirubin >2 mg/dL (0.9%) and ECOG >2 (0.6%).
Conclusions. These data suggest that real-world lymphoma pts are considerably more heterogeneous than clinical trial populations. While the average pt in WCM Database had a lower stage and/or lower prognostic risk score than a typical trial population, over 30% of database pts were > 70, a group that was uncommon in clinical trials. Likewise, almost 30% of Database pts had medical conditions that may have excluded them from clinical trial participation.
Future research should focus on better defining the characteristics and outcomes of pts that either are underrepresented on clinical trials, both intentionally due to eligibility criteria and unintentionally for less clear reasons. It is likely that some eligibility criteria have little impact on treatment and outcomes and may be eliminated from prospective trials, while other trials may focus on pts that remain poorly understood.
Allan:Acerta: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees; Sunesis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Furman:Verastem: Consultancy; Loxo Oncology: Consultancy; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; AbbVie: Consultancy; Acerta: Consultancy, Research Funding; TG Therapeutics: Consultancy; Sunesis: Consultancy; Genentech: Consultancy; Incyte: Consultancy, Other: DSMB; Gilead: Consultancy. Leonard:ADC Therapeutics: Consultancy; BMS: Consultancy; Celgene: Consultancy; United Therapeutics: Consultancy; Biotest: Consultancy; Gilead: Consultancy; Novartis: Consultancy; AstraZeneca: Consultancy; Karyopharm: Consultancy; Genentech/Roche: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; MEI Pharma: Consultancy; Juno: Consultancy; Sutro: Consultancy. Martin:AstraZeneca: Consultancy; Janssen: Consultancy; Kite: Consultancy; Bayer: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal